|
|
Found results for
 trademarks trademarks
1.
|
LYNX


| Application Number |
1670234 |
| Status |
Registered |
| Filing Date |
2022-06-06 |
| Registration Date |
2022-06-06 |
| Owner |
Lyndra Therapeutics, Inc. (USA)
|
| NICE Classes ? |
05 - Pharmaceutical, veterinary and sanitary products
|
Goods & Services
Drug delivery platform technology, namely, orally
administered gastric resident drug delivery forms for the
controlled release of a wide variety of therapeutic agents.
|
2.
|
LYNDRA


| Application Number |
1665039 |
| Status |
Registered |
| Filing Date |
2022-02-01 |
| Registration Date |
2022-02-01 |
| Owner |
Lyndra Therapeutics, Inc. (USA)
|
| NICE Classes ? |
- 05 - Pharmaceutical, veterinary and sanitary products
- 42 - Scientific, technological and industrial services, research and design
|
Goods & Services
Pharmaceutical products, preparations, and substances. Research and development services in the field of dosage
forms for extended release pharmaceuticals.
|
3.
|
LYNX


| Application Number |
219650600 |
| Status |
Registered |
| Filing Date |
2022-06-06 |
| Registration Date |
2024-12-13 |
| Owner |
Lyndra Therapeutics, Inc. (USA)
|
| NICE Classes ? |
05 - Pharmaceutical, veterinary and sanitary products
|
Goods & Services
(1) Drug delivery agents in the form of orally administered gastric resident tablets and capsules for the controlled release of the active ingredients of a wide variety of pharmaceuticals.
|
4.
|

Miscellaneous Design


| Application Number |
1654267 |
| Status |
Registered |
| Filing Date |
2022-02-01 |
| Registration Date |
2022-02-01 |
| Owner |
Lyndra Therapeutics, Inc. (USA)
|
| NICE Classes ? |
05 - Pharmaceutical, veterinary and sanitary products
|
Goods & Services
Pharmaceutical products, preparations, and substances.
|
5.
|

Four triangles and a trapezoid


| Application Number |
217769100 |
| Status |
Registered |
| Filing Date |
2022-02-01 |
| Registration Date |
2024-12-13 |
| Owner |
Lyndra Therapeutics, Inc. (USA)
|
| NICE Classes ? |
05 - Pharmaceutical, veterinary and sanitary products
|
Goods & Services
(1) Pharmaceutical preparations for human use for the treatment of allergies, autoimmune diseases, blood disorders, bone diseases, cardiovascular diseases, cerebrovascular diseases, diabetes, digestive diseases, gastrointestinal diseases, genetic disorders, hepatological diseases and disorders, hematological diseases and disorders, hormonal disorders, kidney diseases, liver diseases and disorders, migraines, headaches, pain, musculoskeletal disorders, ophthalmic diseases, oncological diseases and disorders, pulmonary diseases and disorders, respiratory diseases, renal disease and disorders, skin and nail diseases, and vascular diseases; Antivirals; Pharmaceutical preparations for use in tissue and organ transplantation; Pharmaceutical preparations for the treatment of dermatological diseases and disorders namely dermatitis, skin and bacterial skin infections and fungal skin infections; Pharmaceutical preparations for the treatment of diseases, disorders and infections of the endocrine system, namely growth and thyroid disorders; Pharmaceutical preparations for the treatment of diseases, disorders and infections of the central nervous system namely brain, movement, ocular motility and spinal cord diseases; Pharmaceutical preparations for the treatment of neurological diseases, namely, myasthenia gravis, Alzheimer's, Huntington's Disease, cerebral palsy; Pharmaceutical preparations for the treatment of psychiatric diseases and disorders namely mood disorders, anxiety disorders, cognitive disorders, schizophrenia, and psychoses; Pharmaceutical preparations for the treatment of genitourinary and pelvic diseases, disorders and infections, namely infertility, sexually transmitted diseases, incontinence and sexual dysfunction; Pharmaceutical preparations for the treatment and prevention of HIV infection; Neurological and psychological pharmaceutical preparations for the treatment of drug addictions; Pharmaceutical preparations for the treatment and prevention of HIV infection; Contraceptive preparations; Pharmaceutical preparations for the treatment of malaria; Drug delivery agents in the form of capsules that provide controlled release of the active ingredients for a wide variety of pharmaceuticals.
|
6.
|
LYNDRA


| Application Number |
219076000 |
| Status |
Registered |
| Filing Date |
2022-02-01 |
| Registration Date |
2024-12-13 |
| Owner |
Lyndra Therapeutics, Inc. (USA)
|
| NICE Classes ? |
- 05 - Pharmaceutical, veterinary and sanitary products
- 42 - Scientific, technological and industrial services, research and design
|
Goods & Services
(1) Pharmaceutical preparations for human use for the treatment of allergies, autoimmune diseases, blood disorders, bone diseases, cardiovascular diseases, cerebrovascular diseases, diabetes, digestive diseases, gastrointestinal diseases, genetic disorders, hepatological diseases and disorders, hematological diseases and disorders, hormonal disorders, kidney diseases, liver diseases and disorders, migraines, headaches, pain, musculoskeletal disorders, ophthalmic diseases, oncological diseases and disorders, pulmonary diseases and disorders, respiratory diseases, renal disease and disorders, skin and nail diseases, and vascular diseases; Antivirals; Pharmaceutical preparations for use in tissue and organ transplantation; Pharmaceutical preparations for the treatment of dermatological diseases and disorders namely dermatitis, skin and bacterial skin infections and fungal skin infections; Pharmaceutical preparations for the treatment of diseases, disorders and infections of the endocrine system, namely growth and thyroid disorders; Pharmaceutical preparations for the treatment of diseases, disorders and infections of the central nervous system namely brain, movement, ocular motility and spinal cord diseases; Pharmaceutical preparations for the treatment of neurological diseases, namely, myasthenia gravis, Alzheimer's, Huntington's Disease, cerebral palsy; Pharmaceutical preparations for the treatment of psychiatric diseases and disorders namely mood disorders, anxiety disorders, cognitive disorders, schizophrenia, and psychoses; Pharmaceutical preparations for the treatment of genitourinary and pelvic diseases, disorders and infections, namely infertility, sexually transmitted diseases, incontinence and sexual dysfunction; Pharmaceutical preparations for the treatment and prevention of HIV infection; Neurological and psychological pharmaceutical preparations for the treatment of drug addictions; Pharmaceutical preparations for the treatment and prevention of HIV infection; Contraceptive preparations; Pharmaceutical preparations for the treatment of malaria; Drug delivery agents in the form of capsules that provide controlled release of the active ingredients for a wide variety of pharmaceuticals. (1) Medical and pharmacological research and development services in the field of drug delivery agents for regulating dosage forms for extended release pharmaceuticals; Pharmaceutical research and development services, namely, research and development in the field of drug delivery agents for the extended release of the active ingredients for a wide variety of pharmaceuticals.
|
7.
|
LYNX


| Serial Number |
97166586 |
| Status |
Pending |
| Filing Date |
2021-12-10 |
| Owner |
Lyndra Therapeutics, Inc. ()
|
| NICE Classes ? |
05 - Pharmaceutical, veterinary and sanitary products
|
Goods & Services
Drug delivery systems in the form of oral capsules that provide controlled release of the active ingredients for a wide variety of therapeutic agents
|
8.
|

Miscellaneous Design


| Serial Number |
97085892 |
| Status |
Pending |
| Filing Date |
2021-10-21 |
| Owner |
Lyndra Therapeutics, Inc. ()
|
| NICE Classes ? |
05 - Pharmaceutical, veterinary and sanitary products
|
Goods & Services
House mark for a full line of pharmaceutical products, preparations, and substances
|
9.
|
LYNDRA


| Serial Number |
90879064 |
| Status |
Pending |
| Filing Date |
2021-08-12 |
| Owner |
Lyndra Therapeutics, Inc. ()
|
| NICE Classes ? |
05 - Pharmaceutical, veterinary and sanitary products
|
Goods & Services
House mark for a full line of pharmaceutical products, preparations, and substances
|
10.
|

Miscellaneous Design


| Application Number |
1555308 |
| Status |
Registered |
| Filing Date |
2020-07-31 |
| Registration Date |
2020-07-31 |
| Owner |
LYNDRA THERAPEUTICS, INC. (USA)
|
| NICE Classes ? |
- 05 - Pharmaceutical, veterinary and sanitary products
- 42 - Scientific, technological and industrial services, research and design
|
Goods & Services
Pharmaceuticals for the treatment of neurological and
metabolic related diseases and disorders; pharmaceutical
preparations and substances for the treatment of cognitive
disorders; pharmaceuticals for the treatment of viral,
endocrine, musculoskeletal, cardiovascular, cardiopulmonary,
genitourinary, sexual dysfunction, oncological,
hepatological, ophthalmic, respiratory, neurological,
gastrointestinal, hormonal, dermatological, psychiatric,
drug addiction, substance abuse and immune system related
diseases and disorders; pharmaceuticals for the treatment of
mosquito-borne illnesses; antibiotics; pharmaceutical
preparations and substances for the treatment of infectious
diseases, blood disorders, pain, inflammation, sepsis,
alopecia and obesity; pharmaceuticals for immunosuppression,
namely, solid organ transplant; contraceptive preparations;
pharmaceuticals for the prevention of opioid toxicity or
overdosage, chemical or nerve agent toxicity and radiation
poisoning or sickness. Research and development services in the field of dosage
forms for extended release pharmaceuticals.
|
11.
|
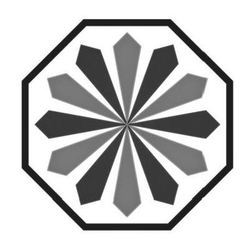
Miscellaneous Design


| Serial Number |
88980899 |
| Status |
Registered |
| Filing Date |
2020-04-21 |
| Registration Date |
2021-06-22 |
| Owner |
LYNDRA THERAPEUTICS, INC. ()
|
| NICE Classes ? |
- 05 - Pharmaceutical, veterinary and sanitary products
- 42 - Scientific, technological and industrial services, research and design
|
Goods & Services
Pharmaceuticals for the treatment of neurological and metabolic related diseases and disorders; pharmaceutical preparations and substances for the treatment of cognitive disorders Research and development services in the field of dosage forms for extended release pharmaceuticals
|
12.
|
LYNDRA


| Application Number |
017452566 |
| Status |
Registered |
| Filing Date |
2017-11-10 |
| Registration Date |
2018-03-08 |
| Owner |
Lyndra Therapeutics, Inc. (USA)
|
| NICE Classes ? |
- 05 - Pharmaceutical, veterinary and sanitary products
- 42 - Scientific, technological and industrial services, research and design
|
Goods & Services
Pharmaceuticals for the treatment of viral, metabolic, endocrine, musculoskeletal, cardiovascular, cardiopulmonary, genitourinary, sexual dysfunction, oncological, hepatological, ophthalmic, respiratory, neurological, gastrointestinal, hormonal, dermatological, psychiatric and immune system related diseases and disorders; antibiotics; pharmaceutical preparations and substances for the treatment of infectious diseases, blood disorders, pain, inflammation, sepsis, alopecia, obesity and cognitive disorders; pharmaceuticals for immunosuppression, namely, solid organ transplant. Research and development services in the field of a platform for extended release pharmaceuticals.
|
13.
|
LYNDRA


| Serial Number |
87982866 |
| Status |
Registered |
| Filing Date |
2017-07-05 |
| Registration Date |
2020-04-28 |
| Owner |
LYNDRA THERAPEUTICS, INC. ()
|
| NICE Classes ? |
05 - Pharmaceutical, veterinary and sanitary products
|
Goods & Services
Pharmaceuticals for the treatment metabolic related diseases and disorders
|
14.
|
LYNDRA


| Serial Number |
87981888 |
| Status |
Registered |
| Filing Date |
2017-07-05 |
| Registration Date |
2019-10-22 |
| Owner |
LYNDRA THERAPEUTICS, INC. ()
|
| NICE Classes ? |
- 05 - Pharmaceutical, veterinary and sanitary products
- 42 - Scientific, technological and industrial services, research and design
|
Goods & Services
Pharmaceuticals for the treatment of neurological diseases and disorders; pharmaceutical preparations and substances for the treatment of cognitive disorders Research and development services in the field of dosage forms for extended release pharmaceuticals
|
15.
|
LYNDRA


| Serial Number |
87516649 |
| Status |
Registered |
| Filing Date |
2017-07-05 |
| Registration Date |
2021-11-09 |
| Owner |
LYNDRA THERAPEUTICS, INC. ()
|
| NICE Classes ? |
05 - Pharmaceutical, veterinary and sanitary products
|
Goods & Services
Pharmaceuticals for the treatment of psychiatric related diseases and disorders
|
|
 trademarks
trademarks










